CRISPR: From promise to practice
According to a report by the Future Today Institute, the size of the global market for CRISPR technologies is projected to exceed $4 billion by mid-decade. In the US alone, CRISPR could contribute $19 billion to GDP by 2032.
So, let's explore how this revolutionary biotechnology is reshaping medicine as we know it.
Remember when CRISPR was just a fascinating lab technique? Well, 2024 marked the year this revolutionary gene-editing tool finally entered mainstream medicine, and 2025 is shaping up to be even more transformative.
What is CRISPR?
CRISPR is an acronym for Clustered Regularly Interspaced Short Palindromic Repeats, a family of DNA sequences found in the genomes of prokaryotic organisms like bacteria.
These sequences are part of a microbial immune system that detects and destroys viral DNA during infections, providing a form of acquired immunity. In biotechnology, CRISPR is widely known as the foundation for the CRISPR-Cas9 genome editing system, which allows scientists to modify DNA precisely.
The system includes two main components: the Cas9 protein, which acts as molecular scissors to cut DNA, and a guide RNA (gRNA) that directs Cas9 to specific genetic sequences.
Over the last 10 years, CRISPR-Cas9-based treatments have been tested in various conditions, including cancers, inherited disorders, HIV and diabetes. However, the most significant progress has been seen in blood disorders.
🥇 The development of CRISPR-Cas9 technology earned Emmanuelle Charpentier and Jennifer Doudna the 2020 Nobel Prize in Chemistry for its transformative impact on science and medicine
The first step: Casgevy makes history
The 2023 approval of Casgevy by both FDA and UK regulators marked a watershed moment in medical history.
This first CRISPR-Cas9-based therapy from Vertex targets sickle cell disease (SCD) and transfusion-dependent beta-thalassemia (TDT) by editing the BCL11A gene to trigger the production of fetal hemoglobin, effectively treating these devastating blood disorders.
Data presented at ASH 2024 showed that:
In SCD, 39/42 (93%) evaluable patients (those with at least 16 months of follow-up) were free from vaso-occlusive crises (VOCs) - primary cause of severe pain and emergency hospitalizations in patients - for at least 12 consecutive months.
In TDT, 53/54 (98%) evaluable patients (those with at least 16 months of follow-up) achieved transfusion-independence for at least 12 consecutive months
Both SCD and TDT patients reported sustained and clinically meaningful improvements in their quality of life, including physical, emotional, social/family and functional well-being, and overall health status.
Casgevy is designed as a one-time gene therapy with potentially life-long benefits and while the treatment's effectiveness is remarkable, its $2 million price tag raises crucial questions about accessibility. The process involves complex procedures: extracting blood-producing stem cells, precisely editing them, and carefully reintegrating them into patients. The treatment also requires patients undergo multiple blood transfusions, and a round of chemotherapy before getting the cells, and a month in the hospital afterward.
Given the technological sophistication, high cost, and demands on patients, initial uptake of the CRISPR treatment has been slow. However, clinicians are optimistic that demand will increase as more success stories emerge.
Beyond the cut: Next-generation gene editing
The original CRISPR-Cas9 system was just the beginning.
Today's genetic medicine toolkit has expanded dramatically with more precise and versatile approaches:
Base editing, developed by Harvard's David Liu, enables the alteration of individual DNA letters without cutting the double helix. Beam Therapeutics is already conducting clinical trials for leukemia treatment using this technology, while Verve Therapeutics shows promising results in treating high cholesterol.
Prime editing offers even greater precision, allowing scientists to delete or add small DNA segments with fewer off-target effects than traditional CRISPR. Think of it as a genetic "search-and-replace" function, offering unprecedented control over DNA modifications.
Perhaps most intriguingly, epi-editing has emerged as a way to regulate gene expression without altering the underlying DNA. Companies like Tune Therapeutics and Epic Bio are preparing to test this approach in clinical trials, potentially offering new treatments for previously untreatable conditions.
The in vivo revolution
One of the most exciting developments is the ability to perform gene editing directly inside the body.
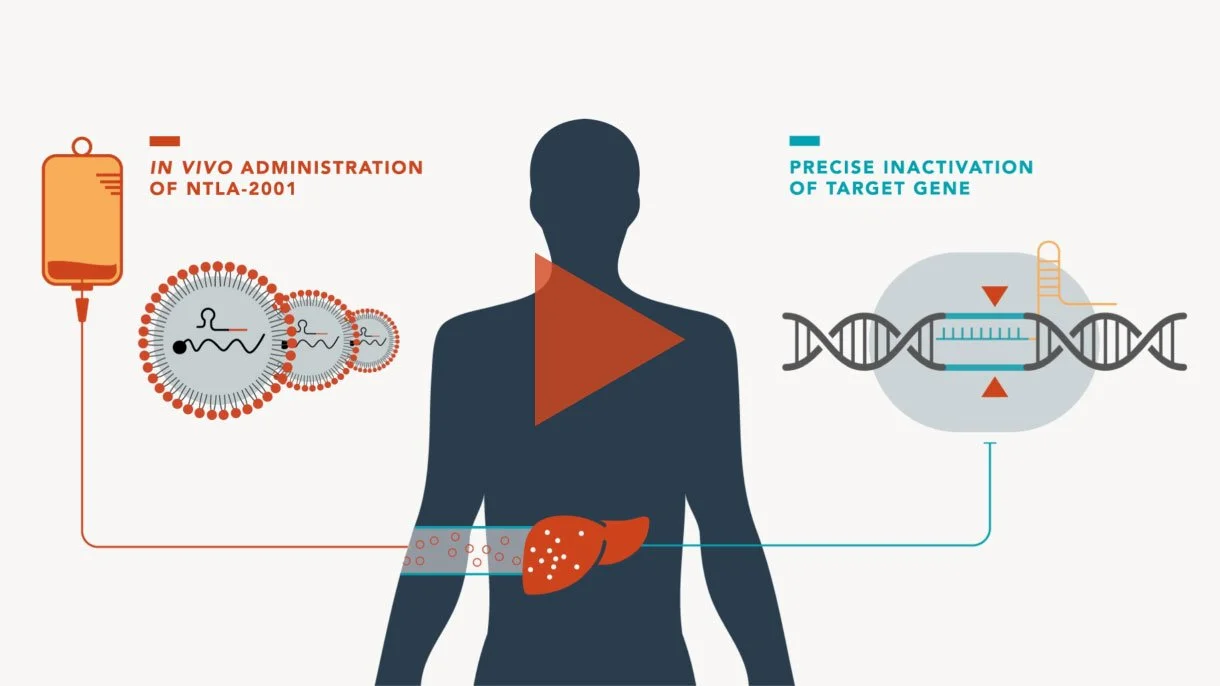
Intellia Therapeutics' NTLA-2001 represents a breakthrough in this area - a single intravenous treatment for transthyretin amyloidosis (a rare, progressive and fatal disease) that targets liver cells to disable disease-causing genes. Early trials have shown remarkable results, with up to 93% reduction in problematic proteins, maintaining stability for at least nine months.
Image from intelliatx.com
This shift from laboratory manipulation to direct body treatment could revolutionise how we approach genetic diseases. Imagine treating cancer not with traditional chemotherapy but with precisely targeted genetic modifications. However, this advancement comes with important considerations about off-target effects and potential impacts on reproductive cells.
🔮 Looking ahead
As we move further into 2025, the convergence of CRISPR with AI promises to accelerate discovery and improve precision. The market reflects this potential, growing from $3.12 billion in 2022 to $4.69 billion in 2024. Yet challenges remain, particularly in making these transformative treatments more accessible.
The question is no longer whether CRISPR will transform medicine and biotechnology but how quickly these transformations will reach everyday practice. As costs decrease and techniques improve, we may be looking at a future where genetic medicine is as common as bone marrow transplants are today.
📫 Want to stay ahead of healthcare innovations? Subscribe to my weekly Healthy Innovations newsletter, where I distill the latest advances in medicine, biotechnology, and digital health into a clear 5-minute briefing delivered straight to your inbox.