How AI is decoding the trillions of bacteria inside you
Let’s explore one of the most fascinating convergences in healthcare today: AI systems that analyze your gut bacteria to predict which foods will spike your blood sugar, which probiotics will actually work for you, and even detect diseases years before symptoms appear.
We're not talking about generic "eat more fiber" advice here. This is AI generating treatment plans tailored to your unique bacterial ecosystem - and it's already delivering measurable results in hospitals right now.
What is AI-powered microbiome medicine?
AI-powered microbiome medicine combines artificial intelligence with gut bacteria analysis to deliver truly personalized treatments. These AI systems, trained on thousands of microbiome samples, can predict how individuals will respond to specific foods, treatments, and disease risks by analyzing the complex interactions within your unique bacterial ecosystem—moving beyond one-size-fits-all approaches to precision medicine at the microbial level.
Your gut contains roughly the same number of bacterial cells as human cells in your entire body—about 37 trillion. These microscopic inhabitants do more than just help digest food; they produce metabolites, communicate with your immune system, and influence everything from mood to medication effectiveness.
The challenge has always been complexity.
Traditional analysis methods simply couldn't handle the staggering interactions between thousands of bacterial species. AI systems, however, excel at exactly this type of pattern recognition in high-dimensional data.
Machine learning models now achieve diagnostic accuracy scores (AUROC) of 0.67 to 0.90 across nine different diseases, while AI systems predict personalized blood sugar responses with 70% correlation accuracy. This performance has been validated across multiple clinical studies.
The diagnostic accuracy score, measured by the Area Under the Receiver Operating Characteristic Curve (AUROC or AUC), reflects a test’s ability to distinguish between diseased and non-diseased individuals, with values ranging from 0.5 (no better than chance) to 1.0 (perfect discrimination). An AUC above 0.80 is generally considered clinically useful, indicating good diagnostic performance.
The market validation is compelling: the global human microbiome market currently sits at $1.2–1.3 billion (2024), with projections reaching $7.2–13.9 billion by 2034 - representing annual growth rates of 18–31%. More revealing is that AI-enabled healthcare startups raise $34.4 million per round on average, an 83% premium over non-AI companies. The economics strongly favor platform approaches over single-asset strategies. Companies developing AI systems that can analyze any microbiome-therapeutic combination command premium valuations compared to those focused on individual microbial drugs.
Here are four ways AI-microbiome convergence is transforming healthcare:
1. Surgical precision in bacterial analysis
AI algorithms are finally handling microbiome complexity that overwhelms human analysis. Deep learning architectures like PopPhy-CNN integrate phylogenetic information to achieve superior accuracy, while transformer-based models like MetaTransformer predict microbial community shifts over time.
The breakthrough involves multi-omics integration using graph neural networks that analyze:
Which bacteria are present in your gut
How they interact with each other
What metabolites they produce
How they respond to different interventions
Random Forest algorithms consistently achieve 0.71-0.94 AUROC scores across studies, while specialized models like WSGMB exceed 0.7 AUROC for predicting colorectal cancer-related bacteria. Recent validation studies demonstrate these models work across 16 different populations from 9 countries - crucial proof they function beyond narrow research settings.
2. Platform companies leading market transformation
The financial landscape clearly reveals which strategic players are emerging as winners.
BioCorteX leads with their CarbonMirror platform, using AI to simulate microbiota-therapeutic interactions and producing over 100,000 previously unknown drug-microbiome interactions through four specialized AI engines.
Persephone Biosciences applies AI and synthetic biology to analyze gut microbiome profiles for predicting which cancer patients will respond to immunotherapy - a crucial advance for precision oncology. Their ARGONAUT study is a longitudinal, prospective, observational investigation enrolling up to 5,000 advanced-stage cancer patients from diverse racial backgrounds. The collected data will be used to develop precision microbiome medicines and identify clinically actionable cancer-specific biomarkers to guide treatment decisions.
Jona secured $5 million to develop proprietary Large Language Models specifically for interpreting individual gut microbiomes, generating personalized scientific literature summaries that translate complex microbial data into actionable health insights.
3. Real clinical applications delivering patient outcomes
GutGPT at Yale New Haven Health helps doctors predict digestive bleeding with remarkable precision. Its diagnostic accuracy is impressive: 97% for blood in stool, 90% for bloody bowel movements, and 86% for vomiting blood. This allows physicians to identify at-risk patients before serious complications develop.
Personalized diabetes management has been revolutionized by AI. These systems learn precisely how your unique gut bacteria influence your blood sugar responses. Rather than generic advice like "avoid sugar," you receive food recommendations tailored to your specific bacterial profile. In clinical testing, these AI systems predicted individual blood sugar responses to different foods with 70% accuracy - making truly personalized diabetes management a reality.
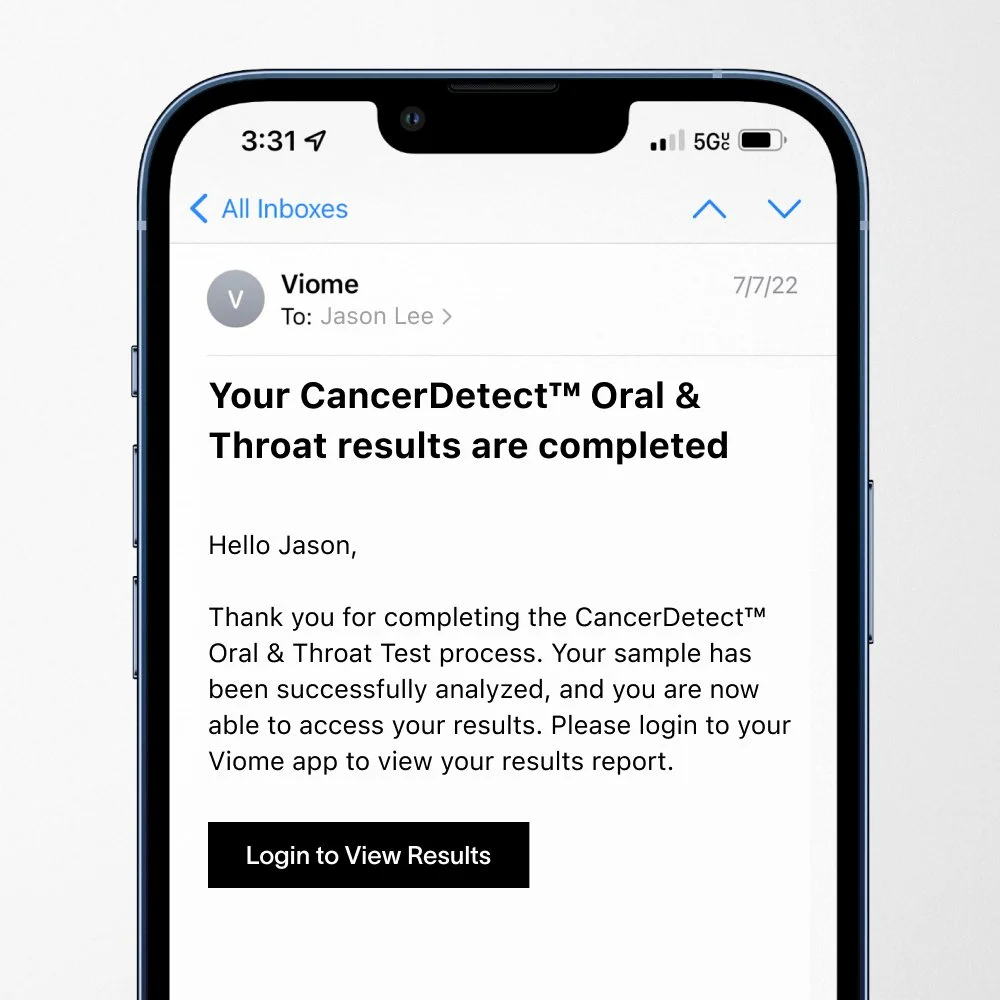
Viome received FDA breakthrough device designation for their AI-powered oral cancer screening platform. Their saliva-based microbiome analysis achieved 83% sensitivity overall and 93% sensitivity for stage 1 cancers with 98% specificity.
4. Technology convergence creating powerful feedback loops
The AI-microbiome revolution gains momentum through integration with wearable technology and synthetic biology. Continuous glucose monitors and smartwatches provide real-time metabolic data that AI systems correlate with microbiome changes, enabling dynamic treatment adjustments.
Food technology companies build personalized nutrition platforms based on these insights - offering specific dietary recommendations tailored to how your unique microbiome metabolizes different nutrients, with adjustments based on continuous monitoring.
Photo by Anna Pelzer on Unsplash
Synthetic biology takes this further with engineered probiotics - designer microbes programmed to produce specific therapeutic compounds based on individual AI analysis. These "living therapeutics" represent a significant evolution beyond traditional probiotics.
This integration extends to everyday medical care through special software that connects your gut bacteria information directly with your doctor's electronic medical records, making it easy for healthcare providers to use this data during your regular checkups.
The road ahead: From breakthrough to standard care
Rapid clinical transformation is already underway. Within the next 2-3 years, the human microbiome-based drugs market will grow from $393.4 million to $1.2 billion by 2030. AI-enhanced clinical decision support systems like GutGPT will expand beyond pilot programs into routine clinical use.
By 2030, machine learning will become pivotal in interpreting microbiome data for customized therapeutic strategies. Personalized microbiome medicine will transition from experimental to standard care. Healthcare systems will integrate AI-microbiome analysis into routine screening protocols, enabling interventions before disease onset.
The challenges remain significant.
Regulatory frameworks are incomplete, technical standardization requires development, and manufacturing live biotherapeutic products under GMP conditions is difficult. Data privacy concerns create additional barriers, especially since microbiome data can uniquely identify individuals with 80% accuracy.
Yet the momentum is undeniable.
With over 150 microbiome-based drugs in clinical trials and proven outcomes already demonstrating value, we're witnessing precision medicine maturing at its most fundamental level - where individual bacterial ecosystems determine therapeutic responses.
The question isn't whether AI-microbiome medicine will transform clinical practice, but how quickly healthcare systems can adapt their infrastructure to harness these capabilities. For the first time, we can truly personalize medicine based on the trillions of microbes that make each person's biology unique.
Join over 550 healthcare leaders who get these insights delivered straight to their inbox! Healthy Innovations is read weekly by executives from AstraZeneca, GSK, Vertex, Roche, and leading healthcare startups, agencies, and investors. Subscribe now to stay ahead of industry trends!